Nootropics: An Emerging Trend in Cognitive Nutrition and Brain Performance
Photo: Pexels
This article originally appeared in Presence Marketing’s October 2021 Industry Newsletter
By Steve Hoffman
From young students, tech professionals, writers and others looking to enhance brain performance, focus and productivity to aging individuals seeking to boost cognitive health, nootropics is emerging as a significant category in dietary supplements. While consumers may not be familiar with the term itself, they are increasingly turning to nootropic products to help with learning, concentration, remembering, recalling information, gaining new skills, alertness, focus and other activities that require cognitive and mental function, says leading natural products market research firm SPINS in its recent The State of Natural report.
Nootropics (sometimes referred to as “smart drugs”) is a catch-all term for products that are reported to enhance brain and cognitive performance, from food and nutritional ingredients to dietary supplement formulations and even some prescription drugs. Taking gingko biloba or even drinking a caffeinated beverage are examples of taking a nootropic to boost memory or enhance alertness and focus.
According to SPINS, sales of functional nootropic ingredients including bacopa, phosphatidyl serine and DMAE all grew over 100% over the 24 weeks ending June 13, 2021. Other nootropic nutritional ingredients seeing sales growth include GABA, L-Theanine, Acetyl L Carnitine, gingko biloba, DHA, medicinal mushrooms, and more.
“A lot of companies are turning to a blend of different nootropic ingredients for a synergistic effect on cognitive health,” noted Scott Dicker, Marketing Data Analyst for SPINS. SPINS also advises retailers that educating yourself and customers is essential. Shoppers may understand what cognitive health is, but educating them and helping them become aware of the wide variety of supplements, foods and beverages that support brain function and cognitive health will help determine their in-store experience, and purchasing decisions, said SPINS.
As of July 21, 2021, “mental complex” supplements experienced a 58% year-over-year growth rate on Amazon, according to ClearCut Analytics in a report in Whole Foods Magazine. Sales have been steadily increasing for these type of supplements since August 2019, and ClearCut Analytics noted that trends often emerge on Amazon before reaching Food/Drug/Mass (FDM). Formulations in capsules are leading sales of mental complex supplements – capsules hold a 71% market share on Amazon, ClearCut Analytics reports.
Today’s aging population is helping drive sales of nootropics by focusing increasingly on brain and mental health, said ClearCut Analytics. SPINS also noted the COVID-19 pandemic has increased overall consumer interest in products for cognitive health.
FMI Survey: 80% of Food Retailers Say Hiring Issues Are Hurting Business
Photo: Pexels
This article originally appeared in Presence Marketing’s October 2021 Industry Newsletter
By Steve Hoffman
Eighteen months into the COVID-19 pandemic, 80% of food retailers surveyed by the Food Marketing Institute (FMI) said difficulties attracting and retaining employees is having a negative impact on their businesses. In its report released Sept. 15, 2021, The Food Retailing Industry Speaks 2021, 42% of retailers surveyed also indicated that supply chain disruptions continue to hurt their businesses. These constraints are happening at the same time that consumer demand for groceries increased 50% in the last year, resulting in unprecedented 15.8% growth in same-store sales, said FMI.
FMI’s 2021 survey represents over 38,000 food retail stores. The survey also found that 95% of food retailers with e-commerce options experienced an increase in online sales in 2020 as a result of changes in consumer behaviors related to the pandemic.
“The pandemic transformed almost every aspect of the food retail industry – from the way consumers shop for groceries and consume their meals to how food is grown, produced and transported to supermarket shelves, to our ability to staff our stores and serve our communities,” said Leslie Sarasin, President and CEO of FMI. “Throughout the past year and a half, the food retail industry has been adapting to meet the shifting needs of the communities they serve. This year’s ‘Speaks’ report outlines the resilience and transformation of the food retail industry amid the COVID-19 pandemic and examines the proactive strategies and investments retailers have made to adapt to the changing food retail landscape.”
“Frontline workers have been lauded as heroes in the face of the pandemic, but recruitment and retention became growing challenges as turnover rose sharply. Retailers have pursued many strategies to resolve these challenges, including increased wages and benefits, flextime and training/skills development”, FMI outlined in a 10 Key Takeaways summary excerpted from the retail report.
Regarding supply chain challenges, FMI said, “Perhaps more than ever before, supply chain is front and center in food retail. Pandemic shortages have led retailers to reassess their supply chains and their engagement strategies with trading partners. Trucking and transportation capacity represents one of the biggest hot-button issues, with some two-thirds of responding retailers saying it is having a negative impact on their businesses.”
Is Organized Crime Responsible for Shrinking Retail Margins and Higher Prices?
Photo: Pexels
This article originally appeared in Presence Marketing’s October 2021 Industry Newsletter
By Steve Hoffman
The answer is yes, according to Kroger CEO Rodney McMullen. McMullen told investors in a quarterly results call on September 10 that its gross margins decreased by 0.6% – and that approximately 25% of that decline was due in part to loss of inventory, or what retailers refer to as shrink. “That's heavily driven by organized crime or at least it appears to be,” McMullen said of the shrink factor, according to the Cincinnati Business Courier. “And I know Congress and other groups are starting to spend more time on understanding what's driving that and what's behind it and what's the distribution channels for the stolen products, as well, and trying to manage that,” McMullen said.
As a result of rising levels of theft, higher supply chain costs and increasing food prices overall, McMullen shared that the grocery chain will raise food prices 2% to 3% this year.
Mark Matthews, VP of Research, Development and Industry Analysis for the National Retail Federation (NRF) told the Cincinnati Business Courier that the organized crime Kroger referred to is not necessarily something involving the “mafia,” but instead comprises organized gangs of people stealing from stores, delivery trucks, warehouses and elsewhere for cash, and it’s a growing trend, he said.
According to NRF’s most recent security survey, 69% of retailers responded that they has seen an increase in organized retail crime. Earlier this year, Home Depot reported that it is using technology to try to curb what it said has become a crime problem as the cost of lumber skyrocketed during the pandemic. Kroger said it is working with trade associations to try to fight the amount of product theft the company is currently seeing.
Tackling food fraud, estimated to cost the food industry as much as $40 billion a year in lost sales, product recalls and legal bills, especially during the pandemic, has been challenging because of complex supply chains and the fact that products can change hands numerous times before they reach supermarket shelves, reported Bloomberg. Cases tagged as fraud, adulteration or authenticity-based jumped 38% in the fourth quarter of 2020, compared to the previous year, reported U.K.-based Food Forensics.
The pandemic has complicated efforts to crack down on such criminal activity, as police resources have been diverted and online marketplaces and delivery platforms are creating more opportunities for illegal goods to be sold, Kimberly Carey Coffin, Global Technical Director at Lloyd’s Register, shared with Bloomberg.
“We are as busy as we have ever been, particularly with white flaky fish, tomatoes, rice and other core commodities that are usually vulnerable to fraud,” Rick Sanderson, Business Development Director of Food Forensics, told Bloomberg.
In examples of the growing problem, the Associated Press (AP) reported in mid-September that four people were arrested on suspicion of stealing nearly $2 million worth of retail products from 43 different stores across California. Investigators found the merchandise the theft ring had stolen stacked “floor to ceiling” inside a mobile home and multiple storage units. In April 2021, police arrested two men and recovered nearly $1 million in goods stolen from grocery stores, AP reported.
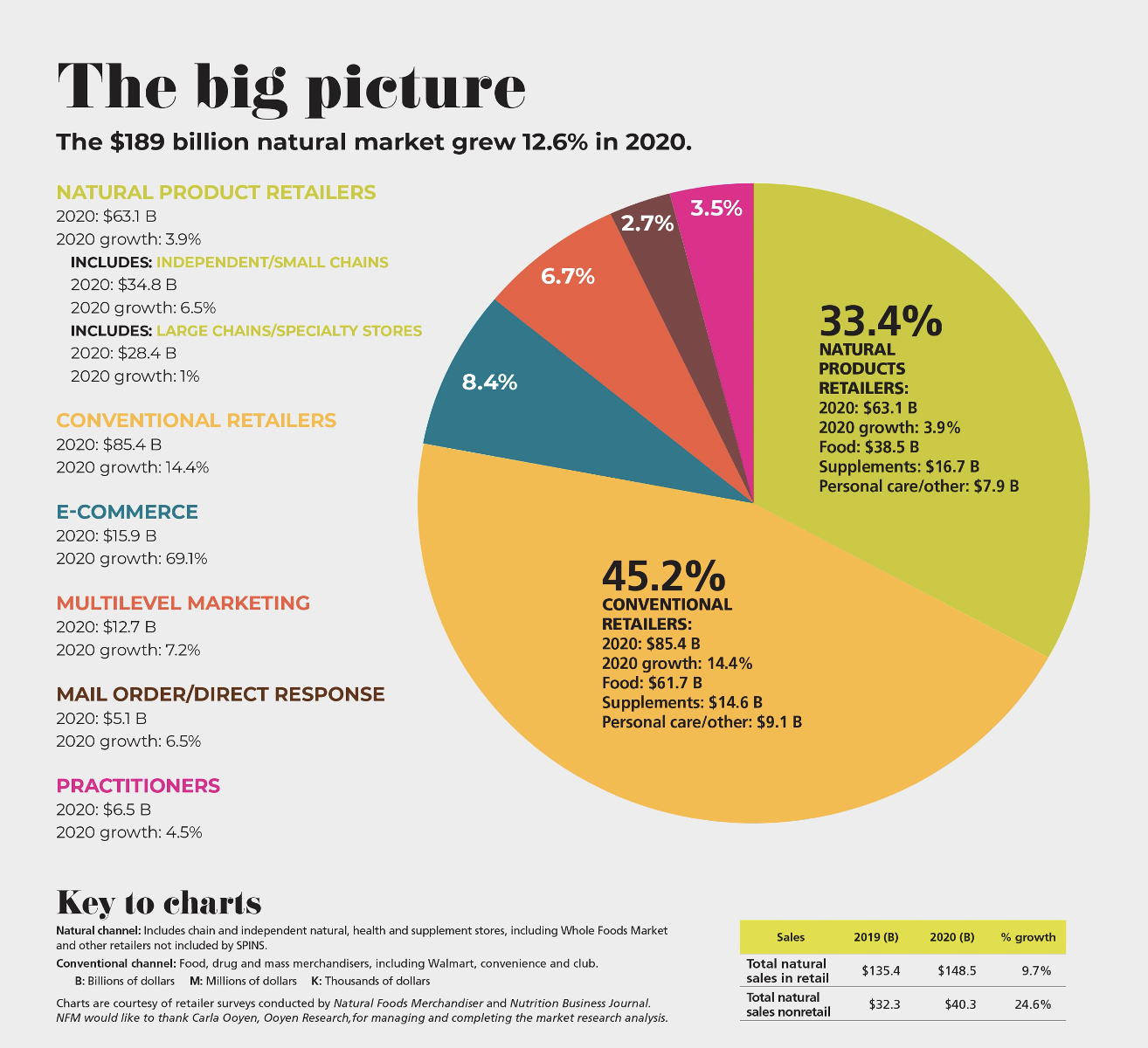
Market Overview: Pandemic Boosts Natural Products Sales 12.6% in 2020
Photo: Natural Foods Merchandiser, New Hope Network
This article originally appeared in Presence Marketing’s October 2021 Industry Newsletter
By Steve Hoffman
Many of the changes independent natural foods retailers adopted during the pandemic, such as digital ordering, curbside pickup, click-and-collect capabilities, traffic flow changes and more, may better help them compete going forward, writes Mark Hamstra in New Hope Network’s 2021 Market Overview Survey. And while natural foods retailers, like retailers everywhere, saw a decline in traffic, the uptick in basket size more than made up for it.
The average sales gain in 2020 among independent retailers surveyed was 6.5%, compared to 1% growth for large natural products chains. In particular, conventional retailers experienced 14.4% sales growth in 2020, and online retailers saw a whopping 69.1% sales growth in 2020. Of note, sales of natural products among health practitioners grew 4.5% in 2020.
Overall, the natural products market, estimated at $189 billion, grew 12.6% in 2020. Product categories leading the growth included meat, fish and poultry, condiments, dairy, fruit and vegetables, breads and grains, packaged/prepared foods and vitamins.
FDA Objects to CBD Being Sold as a Dietary Supplement; Industry Leaders Speak Out
By Steven Hoffman
FDA’s recent decision to reject New Dietary Ingredient applications for full-spectrum CBD from Charlotte’s Web and Irwin Naturals casts a cloud of market uncertainty; passage of Congressional legislation is only option, say industry leaders
Despite months of diligent communications with the U.S. Food and Drug Administration (FDA), along with the submission of volumes of data demonstrating the safety and efficacy of full-spectrum, hemp-derived cannabidiol (CBD), FDA in a letter posted on August 10 rejected two New Dietary Ingredient notification (NDI or NDIN) applications for CBD submitted by pioneering CBD brand Charlotte’s Web and leading natural supplement brand Irwin Naturals.
The decision, based on the agency’s 2020 ruling to treat CBD as a drug, casts a continuing cloud of uncertainty over the market for dietary supplements and functional food and beverage products made with hemp-derived CBD. FDA’s objection only adds to consumer confusion and investor hesitancy, resulting in stunted market growth, say industry leaders, despite rising interest from U.S. farmers to grow hemp and from consumers in using CBD as a safe and effective dietary supplement and herbal remedy alternative.
It was a disappointing, if not surprising, decision by an agency that has historically shown an aversion to dietary supplements and cannabis-derived products, and that has been criticized for being under the outsized influence of the pharmaceutical lobby.
However, given the FDA’s continuing objection to allowing CBD to be sold as a dietary supplement, the only option left is for the hemp industry to advocate for Congressional legislative action, such as H.R. 841 in the House of Representatives and S. 1698 in the Senate, to mandate the FDA to regulate CBD as a dietary supplement and allow for the growth of the emerging hemp-derived CBD market. See U.S. Hemp Roundtable’s legislative guide to take action.
Writing in New Hope Network, Rick Polito reported, “The agency had signaled willingness to work with brands via the NDI process, but in the end appeared intent on delivering a predetermined verdict that CBD, whether as an isolate or as a component of a full-spectrum hemp supplement, is legally identical to the CBD compound as used in Epidiolex, a pharmaceutical drug used to treat epilepsy. The Food Drug and Cosmetic Act ‘exclusionary clause’ holds that supplements cannot contain pharmaceutical ingredients.”
“Why did the FDA put them through the months of doing this dance back and forth?” Steve Mister, CEO of the Council for Responsible Nutrition, asked New Hope’s Polito, emphasizing that Irwin Naturals and Charlotte’s Web were diligent in the NDI process.
“The FDA’s absence, in all measurable forms of leadership, has not only left the CBD market unregulated, it has also cost the hemp industry hundreds of millions if not billions of dollars in lost revenue and investments, and created obstructive barriers and bottlenecks throughout the entire hemp supply chain,” said Morris Beegle, President of We Are for Better Alternatives and producer of NoCo Hemp Expo.
Read on to hear what Charlotte’s Web and other hemp industry leaders and advocates had to say in response to FDA’s decision.
Charlotte’s Web Official Statement
“Today the U.S. Food & Drug Administration (FDA) published an “objection” to Charlotte’s Web’s New Dietary Ingredient notification (NDI) submitted for our full spectrum hemp extract (FSHE), due largely to its drug preclusion provision. This response from the FDA indicates to Charlotte’s Web that without legislation by Congress, this market will remain unregulated…
Over the last 18 months, Charlotte’s Web collaborated with the FDA, providing information about the cultivation, extraction, manufacturing, use and safety behind our proprietary FSHE with naturally occurring levels of CBD. We also supplied research evidencing our FSHE to be different from purified CBD in isolate form which is an FDA-approved drug.
The FDA letter asserts that a FSHE cannot be used in dietary supplements because it is precluded and expresses safety concerns. Regarding safety, the conclusions drawn by the FDA do not appear to be based on the data provided in our NDI application … We requested the FDA correct the record to reflect that data…
The FDA objection to the NDIN does not impact the existing business operations of Charlotte’s Web but does provide useful guidance about what’s required to secure a regulatory framework for FSHE as a dietary supplement.
Both the House of Representatives and the Senate introduced bills that would legislate hemp CBD as a dietary supplement. We believe this legislation is a critical step to protect consumers and to establish guidance for manufacturers, and Charlotte’s Web intends to stay at the forefront of these efforts. Our vertically integrated supply chain and category leadership uniquely position us to work effectively with Congress, and the FDA, to ensure this critical path forward for the hemp industry.”
See Charlotte’s Web’s full statement here.
Jonathan Miller, General Counsel, U.S. Hemp Roundtable, Member-in-Charge, Frost Brown Todd, Washington, DC
“There has been general support for our legislative efforts at U.S. Hemp Roundtable, but there have been some holdouts saying, ‘Let’s give the FDA time to sort it out.’ But this latest NDIN rejection puts that argument to bed. The FDA is clearly not going to take steps to regulate CBD unless Congress tells it to do so. FDA’s objection makes it clear that our top priority is securing passage of legislation such as H.R. 841 and S. 1698.
The U.S. Hemp Roundtable is deeply disappointed to witness FDA’s rejection of two dietary ingredient notifications (NDINs) recently submitted for full-spectrum hemp extracts. FDA’s actions send a discouraging message to the entire hemp and CBD industry, especially in light of the fact that these firms provided more than ample safety data and cooperated with FDA’s requests throughout the process.
When held to the same regulatory standards as other dietary supplements and food ingredients, hemp-derived CBD products have a strong safety profile; the dangers to consumers are only posed by the unregulated marketplace that FDA continues to propagate. This should be a clarion call to Congress that it is time to step in and pass legislation to ensure that CBD products are held to the same standard as all dietary supplements and food ingredients, and to reject an NDIN-only path.
It’s been more than two and a half years since hemp was legalized by the 2018 Farm Bill, and without congressional intervention, the hemp farming industry will continue to struggle, and consumers stand to lose as well.”
See U.S. Hemp Roundtable’s legislative guide to take action.
Janel Ralph, CEO, RE Botanicals and Founder, Harmony CBD
“It is mind blowing to me that FDA is asking us to prove that something is safe when it’s never been proven to be unsafe. It’s FDA’s job to prove it’s unsafe, and it hasn’t been able to do that. The agency claims it’s a drug. Well, at what dose is it a drug? Epidiolex is like 300 mg a day, while full-spectrum supplements are like 25-50 mg a day. FDA could legally make an exemption, but it is choosing not to.
On a personal note, my child Harmony, who was born with Lissencephaly or smooth brain syndrome, has been taking CBD every day of her life for the past seven years. She gets tested regularly for liver enzymes and it has never once affected her liver, yet it has significantly alleviated her suffering from seizures.
At the end of the day, CBD is improving people’s lives across the country and it should be available to everyone as a supplement. FDA needs to start looking at CBD differently. If FDA is going to block something that benefits people, that’s a problem.”
Rachael Rapinoe, CEO and Co-founder, MENDI Co.
“FDA’s objection results in further disconnection from hemp brands, consumers and the education needed to progress the industry as a whole. It shows many of us that the FDA isn’t prioritizing CBD products and bringing a clear path forward in a timely manner. The implications will result in continued confusion and discontinuity of language between brands in the industry.
The FDA is making it increasingly difficult to properly educate and protect consumers from the various types of products on the market and the benefits associated with them. Education is the key to progress and we need the full support of the FDA and medical community if we want to see this industry and its consumers mature.
We will continue to operate in highly restricted grey zones, which is very frustrating. We have a lot research and education to pull real data to educate and empower consumers in the industry. As a brand, we want to protect the public from harmful or dangerous products and guide them in directions that will be more beneficial to their long term health. Also as a brand, we would like to operate in the same capacity as other CPG companies.”
Michael McGuffin, CEO, American Herbal Products Association
“Last month, the U.S. Food and Drug Administration (FDA) replied to two separate new dietary ingredient notifications (NDINs) submitted for ingredients identified as ‘full-spectrum hemp extracts,’ one filed by Charlotte’s Web, Inc., and the other by Irwin Naturals. In its responses, FDA informed both companies that the subject ingredients ‘cannot be used in dietary supplements pursuant to the dietary supplement exclusion provision in 21 U.S.C. § 321(ff)(3)(B)’ on the basis that each qualifies as a ‘CBD product.’
Significantly, the Charlotte’s Web, Inc., ingredient has a cannabidiol (CBD) content of 19.5 mg per serving, and the Irwin Naturals ingredient has a proposed serving limit of approximately 65 mg/day of CBD. In addition, the agency identified ‘concerns about the adequacy of safety evidence’ included in these notifications ‘as a basis for concluding that a dietary supplement containing [the NDI] will reasonably be expected to be safe when used under the conditions’ described in the notifications.
We are fast approaching the three-year anniversary of the enactment of the 2018 Farm Bill, which reflected the decision by the U.S. Congress to support farmers and consumers by establishing a lawful process for production of hemp, which was broadly defined to include the cannabinoids in hemp, including CBD. But ever since FDA has relied on the cited exclusion provision to keep dietary supplements that contain any amount of CBD in a regulatory gray zone, even though the agency already has authority to create a lawful framework for marketing such products.
No one who has been paying attention to this matter should be in the least surprised to see FDA restate its position in these letters. At the same time, it is disappointing and represents another missed opportunity for the agency to bring clarity to the marketplace while using its existing resources to protect the health of the many Americans who already use hemp-derived products.
There are several bills now pending in the U.S. Congress that would resolve this matter and that are supported by the American Herbal Products Association and other organizations who are seeking a resolution that will simultaneously protect the public and the trade. FDA’s NDIN responses should sharpen the focus of all who share such a goal.
At the same time, FDA’s pointed attention to the content of these two NDINs should not surprise any experienced reviewer of the over one thousand such notifications submitted over the past 25 years, and the agency’s replies should be familiar in their scope and tone. Even if Congress acts to remove the current legal barriers to CBD-containing hemp products, companies that intend to bring a new CBD ingredient to market will need to meet the very high standard established for NDINs. In establishing this standard, it is not uncommon for FDA to identify its own specific safety concerns in its response to an initial notification, and the agency often lays out a roadmap for following up with more safety information – as it did for these two full-spectrum hemp extract submissions. These two companies and others who plan to follow their leadership would be well served to study these letters in detail.”
Asa Waldstein, Principal, Supplement Advisory Group; Chair, AHPA Cannabis Committee
“Conducting studies to prove safety is an important part of responsible herbal commerce and Charlotte’s Web should be commended for its time and financial investment. Charlotte’s Web makes the case that a naturally occurring CBD is different from the CBD isolate used in Epidiolex. The FDA comments highlight the agency’s position that any CBD-containing product, including a full-spectrum hemp extract, is not a lawful dietary ingredient due to the Epidiolex drug preclusion provision.
FDA states the Charlotte’s Web (products) are ‘carefully designed to ensure consistent levels of CBD, and that it is produced from your proprietary cultivar (CW1AS1) hemp plants that provide robust levels of CBD.’ FDA’s case here is even though CBD isolate is not added to the products, they still are designed with CBD content in mind. This is a conundrum, as process control and label accuracy are part of dietary supplement regulations. This discussion is further complicated by state requirements in West Virginia and Utah which require CBD content to be listed on the label.
My concern is the FDA response may inadvertently send a ‘do not proceed’ message to companies on the fence about conducting safety studies. I implore companies to continue to add proving product safety into their budgets and strategies.
During this regulatory holding pattern, I suggest companies continue to collect product safety data, as future regulation will likely include a safety component. Acting like a reputable dietary supplement company is the best way forward for hemp-CBD companies. This includes investing in safety studies, but also CFR 111 & 117 compliance, food facility registration, lot number traceability, recall procedures, adverse event reporting, and common allergen labeling.”
Sander Zagzebski, Attorney and Co-leader of Clark Hill LLP’s Cannabis Industry Team
• What does FDA’s objection mean?
“As a technical matter, it means that the person filing the notice (Charlotte’s Web, Inc.) does not have FDA approval to use the dietary ingredient listed in their notification (full spectrum hemp extract) in food products”.
• Why Now?
“The hemp/CBD industry has been operating in a gray area under federal law. While the Farm Bill has legalized certain hemp and hemp derived products, including CBD isolates and full spectrum CBD extracts, under certain circumstances, it is an exaggeration to say the Farm Bill “legalized CBD” in a wholesale fashion. One of the big questions relating to hemp-derived CBD products generally is whether and to what extent manufacturers can include hemp-derived CBD products in food and beverage products that are generally regulated by the FDA. I’m guessing Charlotte’s Web was hoping the FDA under the new Administration would provide some clarity on this issue for the hemp/CBD industry in general and for Charlotte’s Web in particular. By way of background, the law provides that active ingredients in approved pharmaceutical products cannot be sold as dietary supplements in other products. One of the policy purposes behind this law is to encourage companies to undertake the considerable time and expense necessary to get FDA approval for a new pharmaceutical product. If competitors were allowed to sell the active ingredient to a new pharmaceutical product as a dietary supplement, it obviously dilutes significantly the economic benefit of winning FDA approval for a new drug and acts as a strong disincentive to go through the FDA’s drug approval process. When the FDA approved the drug Epidiolex, which is a CBD oral solution for the treatment of epilepsy, that action meant that the active ingredients of Epidiolex, including the CBD compound, could not then be classified as an approved dietary supplement under the law. Left unclear, however, was whether the exclusion would apply only to the specific CBD chemical compound in Epidiolex, or whether it would be applied more broadly to other CBD compounds including ‘full spectrum’ CBD.”
• What was Charlotte’s Web’s objective in applying to the FDA?
“It is likely that Charlotte’s Web was hoping to get clarity on the FDA’s position regarding CBD and to get the FDA’s blessing that, at a minimum, a “full spectrum” hemp-derived CBD products (as opposed to the specific CBD isolate in Epidiolex) would qualify as a permitted dietary supplement.”
• What are the implications going forward re: FDA’s policy toward CBD?
“The broader implications are so far unclear. The hemp-derived CBD industry has existed in this regulatory gray area regarding the FDA for some time, so one could argue that nothing really has changed. On the other hand, the FDA had an opportunity to do the industry a favor, and it declined to do so. Although I don’t have a crystal ball, I think it is likely that the FDA will continue to focus most of its enforcement energy on suppliers that make what the FDA considers to be unsubstantiated health claims, since that doesn’t involve any significant change in their policy stance from the prior Administration. Most federal agencies are loathe to make major policy adjustments when they don’t have a Senate-approved leader at the helm. Since the FDA is currently operating under an Acting Commissioner, it seems a safe bet that the FDA won’t make a major policy decision regarding hemp or CBD until it has its Senate-approved leader.”
• How does that impact companies, consumers and the market?
“In the immediate term, the impact is probably insignificant. The industry had hoped for some clarity, which the FDA has declined to give it, but otherwise the status quo will continue. That said, the industry will have to digest the fact that the FDA hasn’t gone away, and that legislative action is probably necessary to clear the air.”
• What will it take for FDA to allow for and regulate CBD as a safe ingredient in supplements and food and beverage products?
“It is possible that a new FDA Commissioner will, once confirmed, decide to take a more permissive approach to the industry. Absent direction from the top, however, it feels like the career bureaucrats in the FDA do not want to be put in the position of having to make these policy decisions. So legislative action is probably inevitable, eventually.”
• What actions can hemp industry leaders and advocates take to support free access to CBD products in the dietary supplements market?
“Given the regulatory ambiguity, industry leaders would be well advised to be cautious in how they market their products and to be rigid in otherwise complying with all applicable rules and regulations.
• What other comments would you add?
“One coda to this response: The maker of Epidiolex, GW Pharmaceuticals, was sold to Jazz Pharmaceuticals for $7.2 Billion. While GW undoubtedly had other products in the pipeline, the press release announcing the deal describes Epidiolex as GW’s ‘lead product.’ So FDA approval is big business.”
# # #
Talking Online Groceries – Join Jeremiah McElwee, Thrive Market, on Compass Coffee Talk, September 15, 11:30am EDT
Talking Online Groceries: Hear From Thrive Market On What’s Trending Today
Chief Merchandising Officer Jeremiah McElwee from Thrive Market to Share What's Trending in Online Retail on the Next Compass Coffee Talk™
Wednesday, September 15, 11:30 am – Noon EDT
Zoom, Admission is Free
Jeremiah McElwee
Find out what’s trending in the world of online retail and hear key insights from natural products industry leader Jeremiah McElwee, Thrive Market's Chief Merchandising Officer. Jeremiah has twenty-seven years of experience working on countless sides of retail and business, including tackling supply chain issues and growing brands from seed to shelf.
Thrive Market, Inc. was founded in 2014 with a mission to make healthy living easy and affordable for everyone. As an online, membership-based market, Thrive Market delivers the highest quality, healthy and sustainable products at member-only prices, carrying a curation of organic and non-GMO products, and offers 90+ filters and values, allowing you to shop by diet and lifestyle. As a tenured team member at Thrive Market, Jeremiah curated the company’s first catalog, and developed the quality guidelines that still drive product purchasing today.
About Jeremiah McElwee
A twenty seven year veteran of the Natural Products industry, Jeremiah has been on all sides of the business and supply chain, literally from seed to shelf. Part of the initial startup team at Thrive Market, Jeremiah currently serves as the Chief Merchandising Officer there. In addition to developing hundreds of best selling branded products that currently line natural food store shelves and previously managing the Whole Foods & 365 private label brands, he also helped Dr. Andrew Weil develop his branded product platform. When not working as an eco-superhero, he can be found in the Texas Hill Country practicing hot vinyasa yoga, reading children's books to his daughters, or checking the surf report and planning his next escape to the coast.
About Compass Coffee Talk™
Take a 30-minute virtual coffee break with Compass Coffee Talk™. Hosted by natural industry veterans Bill Capsalis and Steve Hoffman, Coffee Talk features lively interactive conversations with industry leaders and experts designed to help guide entrepreneurs and businesses of any size succeed in the market for natural, organic, regenerative, hemp-derived and other eco-friendly products.
Compass Coffee Talk™ is produced by Compass Natural Marketing, a leading PR, branding and business development agency serving the natural and organic products industry. Learn more.
VIEW OUR PAST COMPASS COFFEE TALK EPISODES ON YOUTUBE
Green Jobs: Resources for Jobs & Careers in Sustainable Products
A lot of folks ask us about resources for finding jobs and career opportunities in the $300 billion "Lifestyles of Health and Sustainability" market.
A lot of folks ask us about resources for finding jobs and career opportunities in the $300 billion LOHAS market, i.e., the "Lifestyles of Health and Sustainability" market for natural, organic, eco-friendly, and socially and environmentally responsible products and services. There are a lot of great companies and NGOs in the LOHAS market, from organic food to renewable energy and from yoga to green building. In fact, with significant growth in demand for natural, organic and sustainable products, according to the Organic Trade Association, the organic food industry is creating jobs at a much higher rate than the conventional food industry.
Here are some good resources below for finding jobs in the natural and organic foods and sustainable products industry, and for social and environmental mission based organizations.
Of course, if you identify companies you'd like to work for, check their websites. Often, the larger companies, such as Whole Foods Market, UNFI, Pacific Natural Foods, Earthbound Farm, and other brand leaders will have job postings on their own websites. Do some research of your favorite brands.
We welcome your comments and suggestions to add to the list.
Hope this helps get you started. Happy green job hunting!
Green Job Resources
Green Dream Jobs. You can search by level and region. Awesome resource presented by our friends at SustainableBusiness.com
Luke's Circle is a great resource for sales, marketing, management and executive level jobs in the Denver/Boulder region, created by our friend and colleague Luke Vernon. www.lukescircle.com
GreenBiz has a great sustainable jobs board. http://jobs.greenbiz.com
Just Means job listings have a social mission and NGO focus. http://www.justmeans.com/alljobs
Natural and Organic Industry Careers and Resources. A good compendium of industry resources and job opportunities. http://www.naturalindustryjobs.com
Food Force posts career opportunities with natural, organic, specialty and conventional food companies and brand leaders. http://www.foodforce.com
The Green Jobs Network "empowers people seeking careers in sustainability and environmental responsibility to find jobs, career resources, and build their professional network." http://www.greenjobs.net
Naturally Boulder is another resource for job listings in the Boulder/Denver region. https://www.naturallyboulder.org/resources/jobs/
World Wide Opportunities on Organic Farms. Wanting a Peace Corps-like volunteer experience, but on an organic farm somewhere around the world where you can learn about organic agriculture? Feeling young and adventurous? Check out WWOOF. http://www.wwoof.org
Green Career Guide job thread. http://greencareerguide.jobthread.com
California Certified Organic Farmers, an excellent organization for organic producers, posts job listings. http://www.ccof.org/classifieds.php#emp
ReWork: Founded in 2011 by alumni of the Unreasonable Institute in Boulder, ReWork helps people find careers in values-based, socially responsible and sustainable businesses. http://rework.jobs/talent
Project NOSH: Project NOSH covers the world of entrepreneurial food companies and services that are expanding rapidly due to interest in Natural, Organic, Sustainable, and Healthy (NOSH) products and businesses. Project NOSH helps food and beverage companies to find the right employees, and develop business success. http://www.projectnosh.com/jobs
VeganJobs.com is a free global vegan job and resume hub operated by vegans for vegans and plant-based/vegan-oriented businesses and organizations. https://veganjobs.com/
Food+Tech Jobs: Search for tech, business, design, sales, marketing, operations and PR jobs at leading food companies. https://jobs.foodtechconnect.com/
Food Industry Executive launched its job board in April 2018, and while it is still building steam, it should prove to be a good resource for senior level career opportunities in the food business. http://foodindustryexecutive.com/jobs/
Green Jobs Network contains more than 100 environmental and social impact jobs. If you apply for one of these opportunities, please indicate that you learned about it from Green Jobs Network. https://mailchi.mp/f56a682687be/gjn-july2020
Jobganic: Are you organic, natural, and eco-conscious driven? Looking for jobs opportunities that give you greater satisfaction and that fit your healthy lifestyle? Let recruiters at leading and emerging companies discover you at Jobganic. https://jobganic.com/
SENPA: The Job Board for Natural Products Retailers and Manufacturers. Post a Job. Find a Job. List Your Resume. Find a Candidate. All Natural Products. All in One Place. https://jobs.senpa.org/
One Step Closer: This network of purpose-driven, values-aligned CEOs in the natural products industry offers a job board. Find a job or post a job. https://jobs.osc2.org/jobs/f7222645-9802-4378-a7dd-0ffab0308480
CBD: Consumers Crave Science, Information
This article originally appeared on https://www.letstalkhemp.com/
By Steven Hoffman
Based on a comprehensive consumer survey published in 2020 of 4,000 U.S. consumers plus another 1,000 consumers in Canada, healthy lifestyles research firm Natural Marketing Institute (NMI) found that people are eager and interested in exploring the medicinal value of CBD.
According to NMI’s 2020 North American Cannabis Market Opportunities and Challenges Report, 18% of the U.S. adult population (46 million consumers) say they have personally used CBD products in the past six months.
“Consumers don’t know a lot and they want to learn more,” said Diane Ray, NMI’s Vice President of Strategic Innovation. “Right now, they’re getting information from articles online and family and friends. It doesn’t appear that a lot of authoritative sources are connecting with consumers to provide them with reliable information. It could also be that resistance from social media companies may be a factor in limiting information,” Ray observed.
“There’s an underlying desire to get healthier. People want to believe in the promise of CBD and they want to know how to tap into its benefits. However, the data indicates the industry isn’t educating enough. We see an influx of people experimenting and dabbling with CBD products – from dietary supplements and functional food and beverage to personal care – but that could flatten unless science comes into play more. The market is craving reliable science,” Ray said.
While an older generation in their 60s and 70s are turning to CBD products for pain management – in particular, CBD gummies are number one in terms of consumer choice, according to NMI data – Ray noted that a younger generation, including Millennials and the “i-gen” (the 18-25 age group) are among the most stressed, and they turn to CBD products for anxiety and relaxation.
“Consumers are slowly navigating the puzzle of dosage, quality, etc., to find out what products work for them and fit in their budget,” Ray said. “In the long term, they want to get it where they get everything else, but in the near term, they are looking for education and expertise, and for that they are going to specialty stores, including dedicated CBD stores, dispensaries, drugstores, the internet and natural food stores,” Ray noted.
Natural Marketing Institute identifies five consumer segments that are grouped along the lines of differentiated health attitudes, behavior and psychographics. They include:
Well Beings® – 26%
• Most health pro-active
• Leaders & influencers
• Most multi-cultural
Food Actives® – 14%
• Mainstream healthy
• Basics & balance
Magic Bullets® – 20%
• No healthy lifestyle commitment
• Managers vs. preventative
Fence Sitters® – 23%
• ‘Wannabe’ healthy
• Stressed, want control
• Multi-cultural segment
Eat, Drink & Be Merrys® – 17%
• Least health active
• Taste over health
For more information on the 2020 USA/Canada Whole Cannabis/Whole Health study, visit here.
Zevia Latest in Hot Season of Industry IPOs
This article originally appeared in Presence Marketing’s August 2021 Industry Newsletter
By Steven Hoffman
Zevia the Latest in a Hot Season of Industry IPOs, Private Equity Investment
For decades, the natural products industry has repeated the gospel of the relationship between diet and health.
This year, the Covid-19 pandemic drove that simple truth home, to consumers seeking to boost immunity and health, to businesses seeing opportunity in selling better-for-you products, and to investors, as sales of natural foods and supplements surged in both brick-and-mortar locations and via e-commerce, as shoppers became much more comfortable shopping for groceries from their computers or smartphones at home during lockdown.
Now, as the U.S. healthy food industry emerges from one of the most challenging times in its history, investment in the natural and specialty foods channel is surging, including private equity and public offerings.
This year alone, to date in 2021, more than $10 billion in venture-backed capital has been invested in grocery startups, “vaulting past the $7 billion raised in the sector last year,” reported The Spoon on July 2. According to an April 2021 report by Finistere Ventures and Pitchbook Data, a total of $22.3 billion in private equity was invested in agrifood tech companies, including novel ingredients and alternative proteins, meal kits and food delivery, e-commerce, consumer facing tech, and supply chain, reported Food Dive.
In the public markets, a number of healthy products companies have completed, announced, or are considering IPOs, as the stock market continues to show signs of strength.
Here are highlights of IPO activity announced over the past few months:
Zevia Rides No Added Sugar Trend to IPO
Zero-calorie beverage maker Zevia began trading on July 22 on the NYSE under the ticker symbol ZVIA, pricing shares of its initial public offering at $14 per share. Net sales of Zevia products increased 29% year over year to $110 million, while increasing gross margins from 43% to 45%, reported Food Navigator-USA. Zevia credited its 2020 growth to a combination of velocity gains, growth in e-commerce (now accounting for 13% of sales), and increased distribution, with product available on Amazon, Zevia.com and more than 25,000 retail locations in the U.S. and Canada.
Specialty Grocer The Fresh Market Files for IPO
Specialty grocer The Fresh Market, based in Greensboro, NC, filed for an IPO on July 16. The specialty grocer operates 159 stores in 22 states. In the filing, the company reported comparable store sales growth of 22.3% in FY2020, up from -1.8% the previous year. The company intends to list on the Nasdaq exchange under the symbol TFM. CEO Jason Potter, in a letter to prospective shareholders, said the company made a number of changes after being acquired in 2016 by a private firm, including remerchandising the stores to focus on premium fresh food and offering competitive pricing on staple items, reported Grocery Dive.
Newly Combined Dole plc Announces IPO, Will Trade on NYSE
A newly created company formed from the combination of Total Produce plc and Dole Food Company Inc. announced on July 19 that it is planning an IPO of 26 million shares, priced between $20 and $23 per share. The new Dole plc says it will list shares on the NYSE under the ticker symbol DOLE. The combined businesses each have more than 150 years of history in the fresh produce industry, says the company, which claims it is one of the world’s largest producers of fresh bananas and pineapples, and one of the leaders in value-added salads and fresh-packed vegetables. The company also says it will have a growing presence in categories including berries, avocados and organic produce.
Healthy Snack Company Stryve Foods Begins Public Trading after SPAC Merger
Confident the company can disrupt the meat snack category, Plano, TX-based Stryve Foods, maker of meat snacks, on July 19 announced a merger with Andina Acquisition Corp. III under a shareholder-approved deal. Andina, a specialty purpose acquisition corporation (SPAC), changed its name to Stryve Foods, and began trading on the Nasdaq exchange under the symbol SNAX in late July. Stryve Foods is led by co-founder and co-CEO Joe Oblas, and co-CEO and chief marketing director Jaxie Alt, a veteran of the Dr. Pepper Snapple Group, reported Food Dive. Valued at $170 million, Stryve began by selling air-dried cured meats known as biltong, a tradition originating in South Africa that uses less sugar and additives than traditional meat jerky. Stryve reported sales of $14 million in 2019 and nearly $20 million in 2020, reported the Dallas News.
Instacart IPO Could Be One of the Biggest IPOs of 2021
After a round of fundraising this past spring that valued the company at $39 billion, rumors have abounded about a potential public offering from home delivery service Instacart. That said, Forbes reported in June that if it does go public, Instacart could potentially be one of the biggest IPOs of the year, though it was reported the company could opt for a Direct Listing, an alternative to a public offering in which no new shares are created and only existing, outstanding shares are sold. Currently, there is no information related to an Instacart IPO release date. However, MarketWatch reported that the company replaced its founder Apoorva Mehta as CEO with Facebook veteran Fidji Simo, who will take over leadership of the company in August ahead of an anticipated IPO possibly later this year.
Thrive Market Is Considering $2 Billion IPO Amid Surging eGrocery Sales
Online grocery sales nearly tripled in 2020, according to a July 2021 report by Packaged Facts. Based on this explosive trend, Bloomberg reported on July 8 that online membership-based natural products grocer Thrive Market, headquartered in Los Angeles, is considering an initial public offering at a valuation of more than $2 billion. According to Bloomberg, the company is working with investment bank Goldman Sachs Group. The company was founded by natural products and tech entrepreneurs Nick Green, Gunnar Lovelace, Kate Mulling and Sasha Siddhartha. By 2016, the company raised $141 million across three rounds of funding following its launch in 2014. According to Thrive Market, for every paid membership, Thrive Market donates a free membership to a family in need.
Chobani Files Confidentially for IPO; Company Valuation May Exceed $10 Billion
Chobani, the company that put Greek yogurt on the map, could potentially be valued at more than $10 billion in an IPO, a source told Reuters on July 7. The company on July 6 filed a confidential draft registration statement with the Securities and Exchange Commission (SEC) for a proposed underwritten public offering of common stock, with the number of shared and price range yet to be determined. Chobani, which means shepherd in Turkish, was founded in in 2005 by Turkish immigrant Hamdi Ulukaya, who, with a small business loan, bought a yogurt plant in South Edmeston, NY, that was being closed by Kraft Foods. Chobani, which produces yogurt, oat milk, dairy and plant-based creamers and other products, also is renowned in the natural products industry for its business accelerator program for mission-based food startups, the Chobani Incubator.
Eat Just Is Targeting a $3 Billion Valuation in Considering IPO in Q4 2021
California-based Eat Just, cofounded in 2011 by Josh Balk and CEO Josh Tetrick, has raised a total of $440 million to date, including a recent $200 million placement led by the Qatar Investment Authority, Forbes reported on June 25. According to Forbes, Eat Just’s cultured meat division, GOOD Meat, also secured $170 million in financing in May 2021 as it builds out a large-scale manufacturing facility for cultured meat in Singapore. Eat Just seeks to be one of the first companies to sell meat made from animal cells instead of slaughtered livestock, and according to Forbes. Tetrick feels the positive feedback on cultured meat the company has received in Singapore serves as validation for expansion of cultured meat products in the U.S. in the future. Tetrick confirmed to Forbes that a public offering is “definitely getting closer.”
Naomi Osaka-backed Salad Chain Sweetgreen Confidentially Files for IPO
Fast casual restaurant chain Sweetgreen, specializing in salads and plant-based foods, on June 21 announced it had confidentially filed for an initial public offering, with Reuters reporting that the restaurant chain, which includes tennis star Naomi Osaka as an investor, is hoping for strong investor interest “as demand for plant-based food products surges globally.” The healthy food chain was founded in 2007 with one location in Washington, D.C., by three college roommates at Georgetown University’s business school. Currently, the company operates 122 locations in 12 states across the U.S. To date, the company has raised a total of $478.6 million in funding over 13 rounds, according to Crunchbase. Sweetgreen was named one of Fast Company’s Most Innovative Companies in 2019 and 2020.
Vita Coco, Runa Parent Company Files for IPO with Company Valued at $2 Billion
All Market Inc., parent company of Vita Coco, Runa energy drinks and the water brand Ever & Ever, may be planning an initial public offering as soon as Q3 2021, according to a June 18 report by The Business Times. According to unidentified sources, All Market could be valued at more than $2 billion. Vita Coco, founded in 2004, produces a variety of coconut-based beverages. According to Statista, sales of Vita Coco products totaled approximately $160 million in 2020. All Market Inc. in 2018 acquired Runa, a popular energy drink made from guayusa, found in the Amazon rainforest. The company’s Ever & Ever brand markets pH balanced water in recyclable aluminum bottles.
Oatly Raises $1.4 Billion in May 2021 IPO; Shares Rise 18% on First Day of Trading
Swedish oat milk maker Oatly, which reportedly counts such famous investors as Oprah Winfrey, Natalie Portman, Jay-Z and former Starbucks CEO Howard Schultz, raised $1.4 billion in an initial public offering on May 20. Shares were priced at $17, valuing the company at nearly $10 billion, according to CNBC. The company now trades on the Nasdaq exchange under the ticker symbol OTLY. In 2020, the company reported its revenue more than doubled over the previous year to $421.4 million, with food service accounting for 25% of sales and retail accounting for 75% of sales. Oatly reported a net loss of $60.36 million in 2020 as it invested in expanding into new markets, raising brand awareness and manufacturing, CNBC reported. Oatly CEO Toni Petersson in May told Food Navigator-USA “This is about conversion, it’s about converting people who used to drink cow’s milk into Oatly. And the addressable market is just massive … so it’s growth over profit.” Oatly products are currently sold in 60,000 retail locations and more than 30,000 coffee shops, reported VegNews.
Jessica Alba’s Honest Co. May 2021 IPO Raised $412.8 Million
Honest Co., the cruelty free personal care, household products and baby products brand co-founded by actor Jessica Alba, raised $412.8 million in an initial public offering held on May 4, CNBC reported. Trading under the ticker symbol HNST on the Nasdaq exchange, the company sold 25.8 million shares at $16 per share in its first day of trading, valuing it at $1.44 billion. The Honest Co. had revenue of $250 million in 2016, but has yet to turn a profit, reported LiveKindly. Alba, who started the company in 2011 three years after having her first child, said she founded the brand because she noticed a lack of natural baby products made without harsh chemicals in the marketplace. “When you look at this business, it feels like this is the direction in which the world wants to head,” said Motley Fool analyst Jason Moser on June 28. “A company that's very focused on ESG and sustainability. I think a big question for a business like this, it really boils down to pricing oftentimes. It's a little bit more expensive to make this stuff right now in the near term… As time goes on, those costs will come down, and I think a company like the Honest Co. has some brand equity that could play out in its favor.”
Impossible Foods In Talks to Go Public with a $10 Billion Valuation
Based on an April 8 report in MarketWatch, plant-based foods maker Impossible Foods is preparing for an initial public offering that could value the company at about $10 billion. Founded in 2011 and based in Redwood City, CA, Impossible Foods was recently valued at $4 billion in a private funding round in 2020, MarketWatch reported. The company’s signature product, the Impossible Burger, debuted in 2016. Sources told Reuters in April that Impossible Foods is exploring an IPO within the next year or a merger with a special purpose acquisition company (SPAC). Merging with a SPAC – a shell company that raises funds in an IPO with the goal of acquiring a private company – is becoming a popular alternative for companies seeking to go public “with less regulatory scrutiny and more certainty over the valuation that will be attained and funds that will be raised,” Reuters reported. Reuters also disclosed that Impossible Foods has raised $1.5 billion in the private investments, to date, with backers including Khosla Ventures, Horizons Ventures, and celebrities including tennis star Serena Williams and rapper and music entrepreneur Jay-Z. According to Food Dive, rumors of an IPO for Impossible Foods have circulated since competitor Beyond Meat went public in 2019.
The Secret Sauce Behind a Successful Brand – Join Mike Schall, FocalPoint, on Compass Coffee Talk, August 18, 11:30am EDT
The Secret Sauce Behind a Successful Brand
Former Whole Foods Market Senior Leader of Global Growth and Business Development Mike Schall Will Share Key Components to Success on the Next Compass Coffee Talk™
Wednesday, August 18, 11:30 am – Noon EDT
Zoom, Admission is Free
Mike Schall
Schall is the Managing Director of FocalPoint, a middle-market international investment banking firm, where he also serves as a co-leader for the company’s Food & Beverage practice. Prior to joining FocalPoint, Mike was an integral part of the global senior executive team at Whole Foods Market, where he first served as an advisor to the E-Team and subsequently joined Whole Foods Market as Senior Coordinator, Global Growth and Business Development, providing leadership on a wide range of business initiatives including investments, acquisitions, new ventures, strategic partnerships as well as product development for Whole Foods Market Exclusive Brands.
Mike serves on a number of national boards and organizations and is an active Advisory Board member of Compass Natural Marketing. In addition, Mike has held various C-level positions in food companies for more than 30 years, including Fresh Food Concepts, Monterey Gourmet Foods, Guiltless Gourmet, Townsend’s, David Michael Flavors, Wise Foods, and The B. Manischewitz Company.
About Mike Schall
Mike Schall has successfully navigated significant growth periods, and food recalls; led sales, marketing, culinary, product development, and quality assurance teams; negotiated strategic joint ventures with Fortune 500 companies such as ConAgra and Proctor & Gamble; and served on audit and strategic mergers and acquisitions committees.
Mike Schall graduated from the University of Southern California’s Marshall School of Business with a Master of Business Administration in marketing. He also holds a Bachelor of Arts in marketing from California State University, Los Angeles. He is a frequent speaker at food and beverage industry events and has authored numerous food industry articles and blogs. Mike is an advocate for Atlanta-based OnBoard, a non-profit supporting women in leadership. He is plant-based, an avid runner, and enjoys cooking, reading, and studying history. He and Lisa, his wife of 40 years, have two daughters and three granddaughters.
About Compass Coffee Talk™
Take a 30-minute virtual coffee break with Compass Coffee Talk™. Hosted by natural industry veterans Bill Capsalis and Steve Hoffman, Coffee Talk features lively interactive conversations with industry leaders and experts designed to help guide entrepreneurs and businesses of any size succeed in the market for natural, organic, regenerative, hemp-derived and other eco-friendly products.
Compass Coffee Talk™ is produced by Compass Natural Marketing, a leading PR, branding and business development agency serving the natural and organic products industry. Learn more.